Gas/vapor
hazards usually are present in air. The amount of gas/vapor present reduces
the amount of air present. There are three basic mixtures of a flammable
gas/vapor in air:
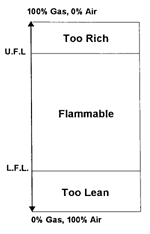
1. Rich mixture: too much fuel; cannot burn
2. Flammable mixture
3. Lean mixture: not enough fuel; cannot burn
Note that the flammable range has an upper limit and a lower limit. Since
the best way to control flammable hazards is to control the amount of fuel
present, we will be focusing on staying below the Lower Flammable Limit.
The Lower Flammable Limit (LFL) is defined as the lowest concentration of
flammable gas or vapor in air sufficient to propagate a flame, given a source
of ignition.
Some people refer to the Lower Flammable Limit as the LEL. Both terms are
used interchangeably, although LFL is the technically correct term.
LFL Monitoring Range
Most flammable gases and vapors have an LFL between 1% and 10%. Let's
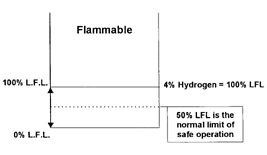
consider hydrogen as an example: hydrogen has an LFL of 4% gas in air.
Another way of saying this is:
4% hydrogen in air = 100% LFL hydrogen
LFL sensors operate from 0 to 100% of the LFL range of flammable gases and
vapors. It is important to note that LFL sensors cannot accurately monitor
mixtures in the flammable range. In fact, most safety rules state that
flammable mixtures be kept below 50% of the LFL. In our example, this means
that the sensor at least should be capable of accurately monitoring from 0
to 2% hydrogen in air (0 to 50% LFL hydrogen).
PPM Monitoring Range
Another way to describe gas concentrations is to convert the gas mixture
into parts-per-million (ppm). For example,
if: 4% hydrogen in air = 100% LFL
hydrogen
and: 4% = 40,000 parts per million (ppm)
then: 40,000 ppm hydrogen = 100% LFL hydrogen
But we do not usually talk about flammable hazards as "parts-per-million"
concentrations. It is much easier and more accurate to define a flammable
hazard as %LFL.
Parts-per-million monitoring is typically conducted in the Low PPM range.
For example, when monitoring hydrogen in a gas cabinet, the full scale range
might be 2,000 PPM hydrogen, which is only 5% of the LFL. Also, PPM
monitoring applications employ different sensor technologies than LFL
monitoring applications.
|
Gas in Air
Mixtures |
|
The Lower
Flammable Limit Range |

|
LFL versus
PPM range monitoring |